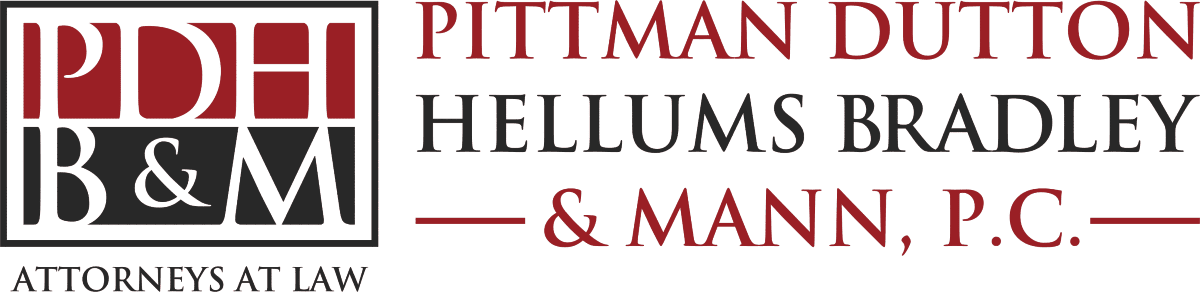Practice Areas
Stryker Hip Replacement Lawsuits
Unfortunately, patients who have received a hip replacement using Stryker Accolade TMZF Titanium hip stems and V40 Femoral Heads may experience severe pain, metal poisoning, tissue death, fractures, falls, loss of mobility and more. Hip implant dissociation, which can happen without warning and occurs when metal parts that form a hip implant separate, can cause severe pain or falls that occur in serious injuries.
In 2014, we reported on a $1.4 billion settlement between Stryker Corp. and thousands of plaintiffs who had unfortunately experienced negative effects from the manufacturer’s Rejuvenate and ABG-II modular-neck stem hip implants. Though the company issued a voluntary recall because of the potential of fretting and corrosion of the device that caused pain and swelling of the hip, the pain and suffering of thousands of patients resulted in a successful lawsuit including more than 5,000 patient lawsuits. Currently, the company is dealing with new lawsuits from victims because of the V40 femoral head. Here’s what you need to know.
Were You Impacted by the Stryker Hip Replacement V40 Femoral Head Recall?
Stryker’s products have continued to pose dangers to orthopaedic patients. In 2016, Stryker issued a recall for their V40 femoral head products, which are the metal ball portions of hip replacements that work with hip stems like the Accolade TMZF and Accolade 2 to replicate the movement of hip joints. Unlike the stem recall, where mailings were sent out to patients alerting them about the recall, this time, Stryker has only contacted surgeons and healthcare facilities, who are placed with the burden of talking with their patients about the recall.
Whether you’ve experienced a hip implant fracture that results in a broken bone around the implant, or you haven’t yet experienced any symptoms, consulting with an attorney if you’ve received a Stryker hip replacement helps ensure you are protected.
What Is Damaging About the Stryker V40 Femoral Head?
Stryker V40 femoral heads are used in metal hip implants, where they are implanted at the upper end of the femur bone and inside the pelvic bone. Stryker issued the recall after receiving high numbers of injury reports. Included in the reports are notices of the V40 femoral heads disconnecting from the implants, which can weaken the system and cause fractures and injuries due to metal debris in the body.
Problems arising because of the V40 femoral head failure, like the Stryker stem failure, can result in bodily injury, hip implant failure and worsened hip condition that requires additional surgery. A subsequent surgery can be very risky, costly and time-consuming because of both the surgery and recovery. Even if you are not currently experiencing any negative symptoms, it is crucial to get help now to prevent future injury.
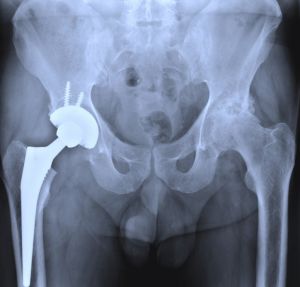
When Is It Time to Hire a Defective Product Recall Attorney?
Because of the history Stryker has regarding lawsuits and patients who have been affected, you may receive some type of offer from Stryker. It is vital to not accept any offer before contacting an attorney, since you may not obtain the just compensation you deserve. There have already been several lawsuits filed against Stryker by patients who have experienced pain and revision surgeries because of the V40 femoral head failure.
After the 2012 recall of Stryker hip replacement stems, Pittman, Dutton, Hellums, Bradley & Mann, P.C. attorney Chris Hellums was appointed to the Plaintiff’s Steering Committee by the judge overseeing all federal lawsuits against Stryker because of the recalled devices. Our law firm has extensive experience helping patients who have unfortunately been victims of defective products manufactured by Stryker.
Your health is your livelihood, and it deserves to be protected. It is our goal to help all patients affected by defective medical products such as these. Call us today at (205) 386-2243 for your free consultation.